Gadobutrol
 | |
|---|---|
| Systematic (IUPAC) name | |
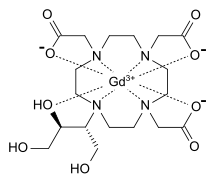
| gadolinium(III) 2,2',2''-(10-((2R,3S)-1,3,4-trihydroxybutan-2-yl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetate | |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Licence data | US FDA:link |
| Pregnancy cat. | C(US) |
| Legal status | POM (UK) ℞-only (US) |
| Routes | IV |
| Identifiers | |
| CAS number | 138071-82-6 |
| ATC code | V08CA09 |
| PubChem | CID 72057 |
| DrugBank | DB06703 |
| UNII | 1BJ477IO2L |
| KEGG | D07420 |
| Chemical data | |
| Formula | C18H31GdN4O9 |
| Mol. mass | 604.710 g/mol |
| SMILES | eMolecules & PubChem |
| | |
Gadobutrol (INN) (Gd-DO3A-butrol) is a gadolinium-based MRI contrast agent (GBCA).
It received marketing approval in Canada and in the United States.
As of 2007, it was the only GBCA approved at 1.0 molar concentrations.
Gadobutrol is marketed by Bayer Schering Pharma as Gadovist, and by Bayer HealthCare Pharmaceuticals as Gadavist.
References
Retrieved from : http://en.wikipedia.org/w/index.php?title=Gadobutrol&oldid=458433501
No comments:
Post a Comment